| Medical Support... |
| ARTICLE |
AUTHOR |
LOCATION |
| Water -- the Base of the Living State and Vital Functions |
V.L. Voeikov |
Faculty of Biology, Lomonosov Moscow StateUniversity, Moscow, Russia. |
| IR-Spectrometry of Water Basis of Biologically Active Substances |
Zubareva G. M. |
Russia, Tver, Tver state medical academy |
| Structural and Dynamical Behaviour of Water in (and on) Nano-confined Systems |
Wilson Quevedo, Rene More, Marcel Petri, Simone Techert |
IRG Structural Dynamics of (bio)chemical Systems, Max Planck Institute of Biophysical Chemistry |
| Collective dynamics in water. Some suggestions on the role of coherent excitations in the living cell and a model of charge density oscillations |
Eugen A. Preoteasa, Marian V. Apostol |
National Institute for Physics and Nuclear Engineering, Bucharest-Magurele, Romania |
| Unexpectedly Critical Role of Hydrophilic Surfaces on Nearby Water |
Binghua Chai, Ivan Klyuzhin, Laura Marshall, Katya Nagornyak, Kate Ovchinnikova, Rainer Stahlberg, Adam Wexler, Hyok Yoo, Qing Zhao and Gerald H. Pollack |
Department of Bioengineering, University of Washington, Seattle WA 98195 |
| Nanomechanics of Exclusion-Zone Water |
Zsolt Mártonfalvi, Miklós S.Z. Kellermayer |
Department of Biophysics, University of Pécs, Faculty of Medicine, Szigeti út 12., Pécs, H7624 Hungary |
| Dissipative Structures in Extremely Diluted Aqueous Solutions of Homeopathic Medicine |
V.Elia,
E.Napoli |
Department of Chemistry University “Federico II” of Naples , Complesso Universitario di Monte Sant’Angelo, via Cintia, 80126 Naples Italy |
| QUANTUM ELECTRODYNAMICAL (QED) COHERENCE AND INTERFACIAL WATER |
Emilio del Guidice |
Istituto Nazionale Fisica Nucleare (INFN) , Milano,Italy and International Institute of Biophysics (IIB) , Neuss,Germany |
| Solute Exclusion from Cells, Gels and Proteins: Relevance to Drug Delivery |
Ivan L. Cameron, Gary D. Fullerton |
University of Texas Health Science Center at San Antonio, San Antonio, TX |
| Terahertz Spectroscopy Reveals Water Helps Proteins Change Shape. |
David L. Shenkenberg |
|
|
|
|
|
|
|
|
"Water -- the Base of the Living State and Vital Functions" by V.L. Voeikov
Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia.
The dominant substance in all the organisms is water. Some hydrobionts where it represents more then 99% by their mass may be considered “living water”. However, to elicit most basic properties of this water making it so special one needs to define what a “living state” is.
More than 70 years ago Russian-Hungarian biologist Ervin Bauer formulated the fundamental principle of Stable Non-Equilibrium to differentiate between animate and inanimate systems: “All and only living systems are never at equilibrium. At the expense of their free energy they ceaselessly perform work against equilibrium, demanded by the physical and chemical laws appropriate to the actual external conditions”. Stable non-equilibrium (excited) state of matter is displayed at all the levels of a living system organization, including the molecular one. Inasmuch as the structure of matter in an excited state differs from structure of the same matter in the equilibrium (ground) state Bauer defined free energy of EXCITED structural elements of the living system (“living matter”) as “structural energy”. Growth and development of a living system goes on due to assimilation of food – conversion of its potential energy into structural energy of “living matter” and transformation of the consumed matter into a non-equilibrium state characteristic of living matter. All forms of work including assimilation of food as are performed by a living system at the expense of structural energy of living matter. To retard spontaneous relaxation of excited state of workable structural elements they should be so interdependent, that a living system may be considered a multilevel coherent system. All vital functions: metabolism and assimilation, multiplication, adaptability, excitability and even evolution may be derived from this principle.
However, Bauer’s theory of a living state does not consider how it could primordially emerge. Besides no conclusive answer is given to the question of what particular substance may stably reside in a non-equilibrium state and return back to it when it releases structural energy for the performance of useful work providing for the continuity of “living matter”.
We’ll reason here that unique properties of interfacial water (EZ-water after G. Pollack) fully meet all the requirements of the Principle of Stable Non-Equilibrium. Most important property of EZ-water is its electron-donating capacity due to much higher state of excitation of electrons in it in comparison to “bulk” water. The natural electron acceptor always present in water is oxygen. If energy of activation even in a form of a triggering stimulus is available EZ-water may donate electrons to oxygen, and the overall reaction of full oxygen reduction may proceed:
2H2O + O2 àO2 + 2H2O + n*hν (Energy)
Though the molecular species at the left and right sites of this equation are the same (water and oxygen) up to 8 eV of energy may be donated by this reaction. It is structural energy (in Bauer’s sense) of EZ-water residing in the stable non-equilibrium state that is released when two water molecules belonging to it (left side of the equation) convert into the ground state water molecules (right side of the equation).
Part of this energy may be used for the restoration of EZ-water. If CO2 and N2 are present in water some part of energy may be used for their excitation and chains of chemical reactions in the course of which complex organic molecules are produced may be initiated. New surfaces turning water into EZ-water appear and the overall stock of structural energy of such aqueous system increases. Thus its ability to perform work against equilibrium enhances.
The necessary condition for this scenario to realize is availability of sufficient energy to keep enough water in a liquid state in which EZ-water (or coherent domains according to E. Del Giudice) and ground state water coexists. This is the necessary condition for spontaneous emergence and sustainability of living systems in all the forms known to us.
|
|
|
|
"IR - SPECTROMETRY OF WATER BASIS OF BIOLOGICALLY ACTIVE SUBSTANCES" - Russia, Tver, Tver state medical academy Zubareva G. M.
Water researches have clearly shown that even ideally pure water represents like difficult arranged system. In what direction as a whole there is a water researching?
From the ecological point of view the water research is directed on studying of its qualitative and quantitative structure. However on a question, whether it is possible to drink water of such structure, we do not receive the answer. It is established, that in the presence of numerous impurity in water their total biological effect surpasses the maximum permissible concentration of separate components established by statutory acts. Thus, it is important to define not only the maintenance of separate substances, but also to register effect of their influence on biological objects, more exact for water component.
The human body almost on 80 % consists of water. However, at studying of pathological conditions arising in a human body, scientists try to find out, what of biochemical tests bears responsibility for it. And again we see, that scientists try to dismember difficult system to the simple parts. And can, it is necessary to reflect, what occurs during this moment to the aqueous component?
Our scientific interest is study the modelling solutions and biological systems. In the researches we have applied the phenomenological approach to studing object that assumes substance studing as a single whole, without separation of primary elements. Novelty of such approach is defined by this: water systems are analyzed as a unit, and their dynamic features are considered. It became possible with introduction in research practice developed infra-red (IR) spectrometer «IKAR» and application the multidimensional analysis of databases by criteria Machalanobis and Bartlett. Such approach to the analysis of the water systems reflecting their complete condition, has allowed us to be engaged in researches of properties of high dilution by pure water the solutions of biologically active compounds.
In the message we will speak on features of the device and application possibilities «IKAR» in researches of a water basis of modelling solutions of biologically active substances and biological liquids. We will present results of experiments. The obtained data will promote clearing-up of mechanisms of participation endogenous waters in functioning of biological systems.
|
|
|
|
"Structural and Dynamical Behaviour of Water in (and on) Nano-confined Systems" by Wilson Quevedo, Rene More, Marcel Petri, Simone Techert
IRG Structural Dynamics of (bio)chemical Systems, Max Planck Institute of Biophysical Chemistry
Time-resolved spectroscopic investigations as well as investigations based on time-resolved x-ray diffraction techniques reveal new insides into the structural dynamics of matter. In this contribution we will present our studies on nanoconfined media like ternary liquid crystal systems (as membrane model systems), or proteins. We will discuss how water solvation shells (in the case of proteins) or the water (structure) itself influences the kinetics and dynamics of the systems mentioned.
Special emphasis will be focused on the ultrafast structural response function of water as been investigated at the Free Electron Laser Facility FLASH at DESY. The remarkable coherent and brilliant properties of this source (100 % transversal coherence, up to 1012 photons / pulse) - combined with its time resolution (30 – 100 fs) provide a unique opportunity to elucidate short living structural intermediates and states. The experiments carried out were based on a pump / probe scheme where a femtosecond optical laser excites the system of investigation and the FLASH soft x-ray pulse (7 nm) probes its structural response function by XUV diffraction techniques. The available energy range (EUV-Soft X-rays) makes it furthermore possible to investigate fundamental processes, such as inner shell photon ionization or the structural response function of matter under extreme laser fields. Early intermediates of radiation chemistry (like solvated electrons) have been observed by applying the experimental scheme of FLASH pump / optical laser probe in the ultrafast time domain.
|
|
|
|
"Collective dynamics in water. Some suggestions on the role of coherent excitations in the living cell and a model of charge density oscillations" by Eugen A. Preoteasa and Marian V. Apostol National Institute for Physics and Nuclear Engineering, RO-077125, Bucharest-Magurele, Romania.
In the approach of life, the theory of long-range coherence developed by Fröhlich overcomes the fragmentary picture of molecular biology and helps understanding the integrative features of living organisms. An active role therein for water, as opposed to its mere function of a cellular environment, is sustained by models of water collective dynamics consistent to Fröhlich’s scheme and based on H2O electrical properties, as illustrated by the theory of coherence domains (CD) [1].The effective mass (excitation energy) of the water CDs was evaluated to 12.1-13.6 eV, much lower than e.g. the electron mass, and accordingly their de Broglie wavelength will be longer. This allows some new hypotheses.
The minimum volume of the cell. The cell may be looked up as a resonant cavity of volume Vcontaining N water CDs, whose wavefunctions reflect totally on the membrane. Due to the low mass, the critical temperature Tc for the condensation of the above CD boson gas may exceed the ordinary temperature of biological organisms (~310 K), if reasonable values of the CD density is assumed. Then, in agreement to the “metabolism first” hypothesis of Dyson [2] we infer that the living state of a cell is essentially defined by its synchronized metabolic reactions. We further suppose that the dynamical metabolic order requires at least two “free” water CDs per cell (the minimum number consistent to a Bose-type condensation) immersed in the cytoplasm water fraction. Thus for Tc = 310 K, meff = 13.6 eV and N = 2, a minimal volume of a cell is estimated to 1.02 µm3, which is close to the volume of 0.35 µm3 of the smallest known cell, the Mycoplasma, and of a typical prokaryotic cell, e.g. 1.57 µm3 for E. coli (for which, however, N = 3). Probably, the first protocells were of about the same size. In similar conditions, the much larger eukaryotic cells will require 102 – 103 free water CDs.
The maximum cell volume. We consider the translation of a water CD with meff = 13.6 eV in a cell described as a spherical well of radius a with impenetrable walls (infinite potential barrier) and neglect the CD orbital movement. The translation energy of the CD is quantized and, assuming the second level E2 to be thermally inaccessible from the first level E1 in the living state at 310 K, a maximal radius of 1.03 µm, i.e. a maximum volume of 4.45 µm3 was estimated. This is confirmed for prokaryotic cells, while in eukaryotic cells, such small volumes are defined by organelles which divide the cell space. The results sustain the evolutionary internalization of organelles as small foreign cells.
A model for liquid water based on plasmon-like excitations [3]. It is suggested that the dynamics of water has a component consisting of O–2z anions and H+z cations, where z is a (small) reduced effective electronic charge. Thus long-range Coulomb potentials in addition to short-range potentials lead to a H+z – O–2z two-species ionic stable plasma. As a result, two branches of eigenfrequencies appear, one corresponding to plasmonic oscillations and another to sound-like waves. Assuming ~1013 s–1 for the plasmonic mode we get z ~0.03. The sound waves are distinctive from the ordinary, hydrodynamic sound, and represent a non-equilibrium elementary excitation whose speed does not depend on temperature (the sound anomaly of water). The plasma oscillations may be quantized; this allowed an order-of-magnitude estimate of the correlation and cohesion energy (vaporization heat) of water. In addition, the oscillating ionic plasma entails oscillations of the delocalized electron cloud with the same eigenfrequency. In an external field, the latter produce an intrinsic polarizability, consistent to the above z value. The results are generalized for a multi-component plasma, as occurring e.g. in the cell cytoplasm, where many types of ionic groups exist. In the living cell, the water ionic plasma oscillations may interact with fields of biomembranes, biopolymers and water coherence domains; as such, they may play a role in cellular communications. In addition the water ionic palsmons should have a very low effective mass, of ~200z meV, and very large de Broglie wavelength, and one can speculate about the possible entanglement of their wavefunctions. This might provide support for intercellular correlations at very long distance, of interest for phenomena occurring in macroscopic organisms, such as embrio-, angio-, and morphogenesis, malign proliferation, contact inhibition, etc.
1. G. Preparata, QED coherence in matter, World Scientific, Singapore-New Jersey (1995); E. Del Giudice et al., Nucl. Phys. B275 (1986) 185;
E. Del Giudice et al., Phys. Rev. Lett. 61(1988) 1085.
2. F. Dyson, Origins of Life, 2nd edition, Cambridge University Press (2000).
3. M. Apostol, E. Preoteasa, arXiv 0803.2949v1, 20 March 2008.
|
|
|
|
"Unexpectedly Critical Role of Hydrophilic Surfaces on Nearby Water" by Binghua Chai, Ivan Klyuzhin, Laura Marshall, Katya Nagornyak, Kate Ovchinnikova, Rainer Stahlberg, Adam Wexler, Hyok Yoo, Qing Zhao and Gerald H. Pollack, Department of Bioengineering, University of Washington, Seattle WA 98195
The impact of surfaces on the contiguous aqueous phase is generally thought to extend no more than a few water-molecule layers. We find, however, that colloidal and molecular solutes are profoundly excluded from the vicinity of various hydrophilic surfaces, to distances typically several hundred micrometers. Such large exclusion zones have been observed next to many surfaces, including hydrogels, biological tissues, hydrophilic polymers, monolayers, and ion-exchange beads. And, many diverse solutes are excluded. Hence, the exclusion phenomenon appears to be quite general.
Several methods have been applied to test whether the physical properties of the exclusion zone differ from those of bulk water. NMR, infrared, and birefringence imaging, as well as measurements of electrical potential, viscosity, and UV-VIS absorption spectra, reveal that the solute-free zone is a physically distinct, less mobile, ordered, phase of water that can co-exist essentially indefinitely with the contiguous solute-containing phase. Indeed, this unexpectedly extensive zone may be a candidate for the long-postulated “fourth phase” of water.
The energy responsible for building this charged, low entropy zone may come from sunlight. We found that incident radiant energy including all visible and near-infrared wavelengths induce exclusion-zone growth in a spectrally sensitive manner. IR is particularly effective. Ten-minute exposure to LED radiation at 3.1 µm (corresponding to OH stretch) causes exclusion-zone-width increase up to four times. Apparently, incident photons cause some change in bulk water that predisposes constituent molecules to reorganize and build the charged, ordered exclusion zone.
Photons from ordinary sunlight, then, may have an unexpectedly powerful effect that goes beyond mere heating. It may be that solar energy builds order and separates charge between the exclusion zones next to hydrophilic surfaces and the bulk waters beyond — each separation creating a battery. The resemblance to photosynthesis is evident. Indeed, this light-induced action would seem relevant not only for photosynthesis but also for all realms of nature involving water and interfaces.
1. Zheng, J.M. and Pollack, G. H.: Long range forces extending from polymer surfaces. Phys Rev E.: 68: 031408, 2003.
2. J.M Zheng, W.-C Chin, E. Khijniak, E. Khijniak, Jr, and G. H. Pollack: Surfaces and Interfacial Water: Evidence that hydrophilic surfaces have long-range impact. Adv. Coll. and Interface Sci, 127: 19-27, 2006.
|
|
|
|
"Nanomechanics of exclusion-zone water" by Zsolt Mártonfalvi and Miklós S.Z. Kellermayer* Department of Biophysics, University of Pécs, Faculty of Medicine, Szigeti út 12., Pécs, H7624 Hungary
Intra- and extracellular space is rich in charged biopolymeric surfaces. A vast array of previous studies suggest that the properties of water at and near these surfaces are quite different from that of ordinary bulk water: the ordering of water molecules in co-ordinated multiple layers results in the exclusion of solutes, and thereby leads to the formation of an exclusion zone near the surface. Recent experiments revealed that even large, micron-sized charged particles can be excluded from the vicinity of charged polymeric surfaces to distances reaching hundreds of microns. The exclusion is probably driven by long-range forces, the nature and origin of which remain to be understood.
To investigate the mechanisms of solute exclusion, we combined microfluidics with optical trapping nanomechanics. We followed the collective motion of latex beads (0.5 - 2.0 µm diameter) suspended in aqueous solutions and introduced near the surface of Nafion (synthetic, perfluorinated teflon) in a vertically-mounted sample chamber. Following the entry of the suspension in the chamber, the beads began to rise and move collectively away from the Nafion surface, driven by the vectorial sum of gravitational, buoyancy, frictional and exclusion forces. The velocity of the bead phase boundary decayed exponentially as a function of time. In pure water the time constant of velocity decay was ~100 s, and the exclusion zone grew to a width of ~200 µm. From the initial velocity of ~2.3 µm/s we calculated an exclusion force of ~35 fN acting on a single, 1 µm bead. Replacing Nafion with Parafilm completely abolished bead exclusion. Replacing pure water with ethanol solution reduced the width of the exclusion zone. We tested four possible mechanisms that may lead to the generation of the exclusion force:
1) direct pressure by polymer strands dissociating via reptation,
2) direct pressure by entropic polymer brush,
3) direct force applied by moving phase boundary,
4) physical-chemical gradient that manifests in force.
We excluded the reptation and entropic brush mechanisms by imaging and mechanically tapping the Nafion surface with atomic force microscopy. To distinguish between the moving phase boundary versus gradient mechanisms, we trapped beads with optical tweezers in order to relocate them between phases. When beads held, with optical tweezers, in the exclusion zone were released by turning the laser off, they started moving away from Nafion and soon caught up with the the bulk of the beads. Thus, it is not a translocating phase boundary that pushes the beads away from the polymer surface. Rather, a physical-chemical gradient is present within the exclusion zone that sustains a distance-dependent force. In sum, the properties and dynamics of water in the vicinity of biolymer surfaces may have direct mechanical consequences. How they might influence biologically important processes such as molecular recognition, enzymatic activity and motor protein function, remain to be resolved.
|
|
|
|
Dissipative Structures in Extremely Diluted Aqueous Solutions of Homeopathic Medicine V.Elia and E.Napoli Department of Chemistry University “Federico II” of Naples , Complesso Universitario di Monte Sant’Angelo, via Cintia, 80126 Naples Italy.
In the last decade, we have investigated, from physicochemical point of view , whether water treated by the procedure of homeopathic medicine( leading inexorably to systems without any molecule different from the solvent) results in water different from the initial water.
The answer, unexpectedly , but strongly supported by many experimental results is positive. We used well established physicochemical methodology : flux calorimetry, conductometry, pHmetry and galvanic cells electrodes potential. unexpectedly, the physicochemical parameters evolve in time.
The water solvent exhibits large changes in measurable properties as a function of its history, the solute previously dissolved, and time. In particular we found evidence of two new phenomena, both totally unpredicted, in homeopathic dilutions: the presence of a maximum in the measured physicochemical parameters vs sample age, and their dependence on the volume in which the dilution is stored. These new experimental results strongly suggest the presence of an extended and ordered dynamics involving liquid water molecules.
|
|
|
|
"QUANTUM ELECTRODYNAMICAL (QED) COHERENCE AND INTERFACIAL WATER" Emilio del Guidice Istituto Nazionale Fisica Nucleare (INFN) , Milano,Italy and International Institute of Biophysics (IIB) , Neuss,Germany
It has been shown in the last 20 years in the conceptual framework of QED that liquids are two-fluid system.The first fluid is an ensemble of Coherence Domains (CD),where the component molecules oscillate in unison between two configurations of their electron cloud in tune with an e.m. field self-trapped in the CD. The second fluid is a dense gas of independent molecules, put out of tune by the thermal fluctuations, which is trapped in the interstices among CDs. The interplay between e.m. and thermal fluctuations produces a flickering mixture of the coherent and noncoherent fractions of the liquid.Water is peculiar since the coherent oscillation in the CDs raises the component molecules up to the threshold of ionization, so that in each CD there is a plasma of quasi-free electrons , whose excitations give rise to a spectrum. It is this possible to induce CD oscillations able to produce a coherence among CDs , that can reach macroscopic sizes.A surface is able to interact with water CDs producing a further stabilization that shields water CDs at the interface from thermal fluctuations. Interfacial water is thus almost fully coherent and exhibits the corresponding properties:
i) the exclusion of the noncoherent fraction prevents solutes from entering interfacial water (exclusion zone)
ii) the presence of a plasma of quasi-free electrons in the CDs makes interfacial water a donor of electrons, so that it becomes a reducing agent.
CDs , as all coherent systems, are able to add to their chemical potential any externally applied e.m. potential ( Boehm-Aharonov effect) . Then a negatively charged surface lowers the CD chemical potential , producing the crowding of many CDs and the formation of an exclusion zone, whereas the opposite result is produced by a positively charged surface. The emerging QED picture of the interfacial water supports the findings of the Pollack group. An independent electrochemical evidence is presented, that supports such findings.
|
|
|
|
"Solute exclusion from cells, gels and proteins: relevance to drug delivery" Ivan L. Cameron and Gary D. Fullerton, University of Texas Health Science Center at San Antonio, San Antonio, TX 78229
A vital dye exclusion test is the most commonly used method for determination of living vs. dead cells. Cell death is thought to disrupt the vital dye (i.e. methylene blue, trypan blue, nigosin, propidium iodide and others) exclusion function of the cell membrane and this allows the dye to enter and stain the intracellular contents. An alternate dye excluding mechanism is that most if not all of the water in the cytoplasm of a living cell is structured in such a way as to be non-solvent for the vital dye. As proteins are, by dry mass, the most abundant material in the cell it seems logical to think that proteins are the likely source of water structure. According to this idea, death of the cell causes decrease in water structure and its dye excluding properties.
It is difficult to get enough cytoplasm to study its solute exclusion and other physical properties. As reported here, hen egg white has provided a useful surrogate for cytoplasm and can be separated into thin and thick albumen fractions that remain non-miscible. Thick albumen without a membrane is vital dye excluding, demonstrates osmotic behavior and has the ability to transform from a dye excluding gel to a non-dye excluding more fluid sol by pressure agitation a gentle shear force. The sol phase does, with time, transform back to a dye excluding gel. Thin albumen is also shown to have a dye excluding shell of water that can be removed by centrifugal pressure.
It seems likely that protein rich living cells, like thick albumen, would have a proclivity to exist in a vital dye excluding gel state that can transform to a more fluid non-dye excluding sol state upon physical perturbations or death.
It may be that structured cell water excludes drugs from interaction with their cellular target molecules and that physical means could be used to help destructure water and therefore allow access of drugs to their target molecules at specific sites in the body
|
|
|
|
"Terahertz spectroscopy reveals that water helps proteins change shape" by David L. Shenkenberg
Just about everyone takes water for granted. Now, using a technique called terahertz absorption spectroscopy, researchers at the University of Illinois at Urbana-Champaign have shown that water plays a more important role than previously thought.
Among other things, they found that water helps proteins fold into their natural structure, termed the native state. Proteins begin as a long chain of amino acids that folds into a more complex structure, and the way that proteins fold has a profound effect on their activity. However, biochemists often neglect water in their protein folding calculations.
One reason why water has been neglected is because it is transparent and therefore difficult to measure spectroscopically. “What has changed in the last few years is that spectroscopic methods that can actually look at the water have come online,” said chemistry professor Martin Gruebele, who oversaw the investigation.
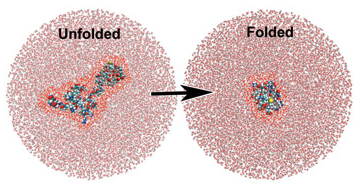
A simulation showing water molecules around an unfolded and a folded protein. The water molecules closest to the protein are highlighted bright red for effect. Terahertz spectroscopy has shown that the water molecules closest to the protein behave differently than the surrounding ones and actually participate in the folding process.
For about the past 20 years, nuclear magnetic resonance spectroscopy has been used to study water molecules, but it can monitor only the surface of the water. To go deeper, scientists had to use terahertz spectroscopy, but terahertz lasers have only recently become powerful enough to get a signal in spectroscopy experiments.
Terahertz means 1 trillion cycles per second, which corresponds to wavelengths from the microwaves to 1 mm long. Recently, terahertz lasers became commercially available.
|
|
|
|
"Slaves to water"
Gruebele and colleagues at the university and at Rurh-University Bochum in Germany used a terahertz spectrometer that they built in the lab to monitor the motion of water and proteins on a picosecond time scale. That’s one-trillionth of a second! They used a protein called ubiquitin, which gets its name because it is ubiquitous in cells, as described in the July 23, 2008, issue of Angewandte Chemie.
When they monitored the motion of the water molecules as ubiquitin folded into its native state, they found that the molecules moved into the natural configuration faster than the protein itself, which supports the work of Hans Fraunfelder at Los Alamos National Laboratory in New Mexico. Fraunfelder has said that the motion of proteins is “slaved” to the faster motion of water molecules.
In general, water around proteins absorbs more of the terahertz radiation than pure water. Gruebele compared the proteins with ice. “The proteins lock in the water like ice and prevent it from absorbing as much light,” he said, whereas the absorption increases once the ice melts and the water molecules are free to tumble around.
The ice analogy mirrors the real environment of the cell. Water in an organism is never more than a few nanometers away from proteins and other large molecules. Much of the water in cells is locked in and around proteins.
|
|
|
|
"Like a fish"
With all the proteins that the researchers have studied to date, the terahertz absorption of the water has decreased when they have used proteins with altered amino acids, and the absorption of water around completely unfolded proteins has looked like that of pure water. From these results, the researchers have concluded that the natural structure of the protein stores the most water. “We have no idea why that is,” Gruebele said.
But he speculates that water might enable some proteins, particularly enzymes, to draw biological molecules toward them. For example, enzymes could pull molecules into their active sites where they can modify the molecular substrates. “They might get sucked into a protein,” he said, “like a fish that sucks in water to get prey.”
Further research in this direction could have exciting applications. “One of the things that might come out of this [research] is that you can tailor the structure of the protein so that its function can be enhanced,” Gruebele said.
“Water can be viewed as a ‘designer fluid’ in living cells,” Gruebele said. But he knows that it may take a while for this idea to gain acceptance. “As you know, this sort of thing takes years and years before minds will eventually change,” he added.
The Anfinsen experiment has become a canonical way to explain protein folding. Christian B. Anfinsen showed that he could unfold a protein by breaking the sulfur-sulfur bonds from the amino acid cysteine and then refold the protein by restoring those disulfide bonds, an experiment for which he won one-half of the Nobel Prize in chemistry in 1972.
Although the Anfinsen experiment showed that the amino acid sequence and disulfide bonds are crucial to the folding process, his work was done in vitro. “If Anfinsen were around now, and he were seeing the water molecules, he might have agreed,” Gruebele said. “He did not intend for this to occur in a vacuum.” Proteins called chaperones also help other proteins fold in the cell, but that’s another story.
|
|
|

